Former team members :
Using chemical Biology, validate PfAM1 as a drug target and develop new strategies for the treatment of malaria.
The WHO/TDR initiative promotes drug target validation for neglected diseases. As such, innovative strategies are being developed to discover new antiplasmodial targets and may include a target-driven approach by cell-based screening, drug repositioning and chemogenomics. In this context, the Plasmodium proteases are putative targets. Their study benefits from the successful work done on proteases in other pathologies, such as AIDS, hypertension and type-II diabetes. Among the metalloproteases of Plasmodium, falcilysin is involved in the intravacuolar digestion of globin and has no reported inhibitor. On the other hand, aminopeptidases degrading oligopeptides resulting from haemoglobin digestion are well-studied. Recently, inhibitors of LAP (Leucine-aminopeptidase), a hexameric aminopeptidase of the M17-family, have been described.
Florent et al characterized the aminopeptidase M1 (PfAM1 or PfM1AAP) that shows a strict aminopeptidase activity at pH 7.4 and a broad substrate spectrum. PfAM1 localizes differently in trophozoites and schizonts, suggesting a particular role in the intraerythrocytic life of the parasite. PfAM1 degrades oligopeptides resulting from haemoglobin digestion either in the vacuole or the cytosol. The inhibition of PfAM1 by bestatin analogues induces swollen digestive vacuoles due to the accumulation of oligopeptides. Also, a role in the reinvasion of erythrocytes has been found. Studies on PfAM1 localization suggest that it is trafficked via the parasitophorous vacuole. PfAM1 is also present in the nucleus, but so far no function can be related to its nuclear localization.
Given these results, PfAM1 can be considered an attractive candidate to initiate screening and lead optimization. Our research group was the first to describe PfAM1 screening and the first two series of hydroxamic non-peptidic non-selective PfAM1 inhibitors with a mixed mode of action in 2003. Since then, we have developed the first selective inhibitors of this enzyme.
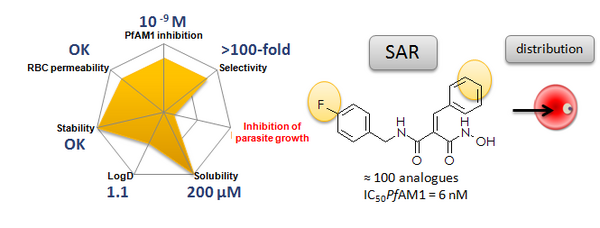
We established in-depth structure-activity relationships of a selective family of hydroxamate PfAM1 inhibitors based on the malonic template. In particular, we performed a “fluoro-scanning” around hit that enlightened the key positions of this halogen for activity. Docking of lead BDM14471 is consistent with in vitro results. The stability of the lead was evaluated in microsomes, plasma and towards glutathione. The in vivo assessment of PfAM1 as a pharmacological target was performed at a molecular level using the nanomolar hydroxamate inhibitor BDM14471 in a distribution study. This study provides important insights for the design of inhibitors of PfAM1, and the validation of this target.