Develop PDE inhibitors as new therapeutic strategies to fight Malaria.
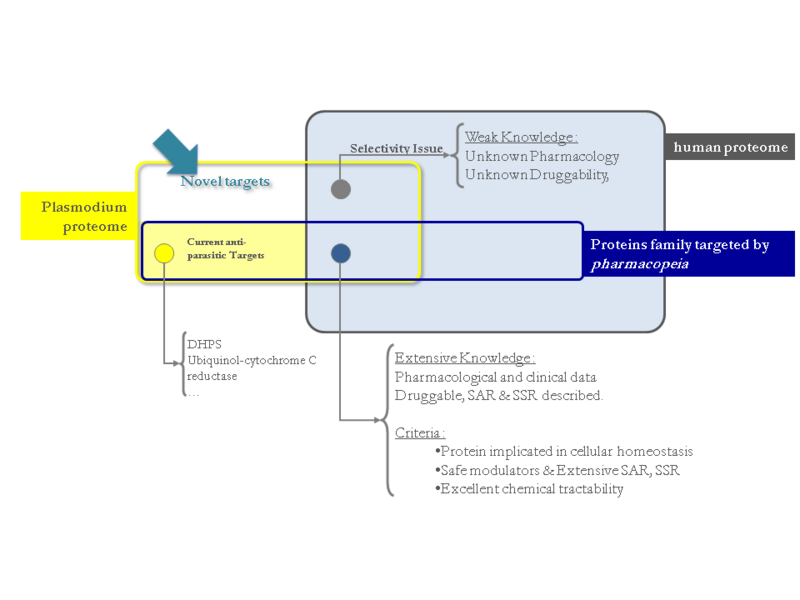
The genome project of P. falciparum has been completed and can now be used to reveal potential new drug targets. Sequence alignments of the PDEs of P. falciparum revealed 4 orthologues of the human PDE, while sequences in regions important for catalytic activity are highly conserved. PDE5 inhibitors such as sildenafil, vardenafil or tadalafil have been developed for the treatment of erectile dysfunction. These PDE5 inhibitors exhibit different PDE selectivity profiles, in particular in their relative affinities for PDE5, PDE6 (found in photoreceptor cells) and PDE11 (of yet unclear biological function). Tadalafil present a good selectivity profile over PDE6.
The synthesis of tetrahydro-beta-carboline derivatives, as tadalafil, is relatively simple, opening access to a variety of rationally designed structural modifications, permitting what we refer as a “molecular morphing” that would change pharmacological properties through a progressive transition.
Using this original “molecular morphing” approach, we have been able to turn PDE5- into P. falciparum PDE -selectivity, and to obtain compounds with in vitro antimalarial activities superior to chloroquine and comparable to artesunate. Our aim is now to produce a pre-clinical drug candidate by optimizing simultaneously multiple properties according to 3 types of requirements: 1) Pharmacokinetics 2) Potency, selectivity and novelty and 3) Target validation.
Malaria is a disease which is caused by parasites of the genus Plasmodium that are spread from person to person through the bites of infected mosquitoes. There are four species of Plasmodium infecting man– Plasmodium falciparum, P.vivax, P.malariae, and P.ovale. P.falciparum is by far the most deadly type of malaria infection. Approximately, 40% of the world’s population are at risk of malaria. Every year, more than 500 million people become severely ill with malaria. Most cases and deaths are in sub-Saharan Africa, and are essentially pregnant women and children. However, Asia, Latin America, the Middle East and parts of Europe are also affected. Travellers from malaria-free regions going to areas where there is malaria transmission are highly vulnerable – they have little or no immunity and are often exposed to delayed or wrong malaria diagnosis when returning to their home country.
If not treated promptly with effective medicines, malaria can cause severe illness that is often fatal. Prevention, early diagnosis and prompt treatment are the basic elements of malaria control, shortening the duration of the disease and preventing the development of complications and the great majority of deaths from malaria. Several drugs, including chloroquine and quinine, are used for the treatment of malaria. However, the rapid spread of antimalarial drug resistance over the past few decades undermines malaria control efforts and is therefore a major public health problem.
Cellular differentiation is an important process in the life cycle of the malaria parasite. Intense asexual divisions occur in the liver and in erythrocytes: the later rounds of asexual replication correspond to the pathogenic phase of malaria.
In this context, cyclic nucleotide signalling, which plays a pivotal role in the growth and differentiation of lower organisms, is essential for their survival. It is regulated through cyclic nucleotide production by adenylate and guanylate cyclases and hydrolysis by cyclic nucleotide PDEs (phosphodiesterases). PDEs from lower single-cell organisms have been reported and revealed to be distinct from the 11 mammalian PDE families. In Plasmodium, the presence, and some physiological roles of PDEs have been predicted, and a first PDE (PfPDE1) of P. falciparum was described in 2005.
In 2004, we hypothesized that PDE inhibitors could have an effect in the cell proliferation of asexual blood parasites, resulting in antimalarial activity, and that marketed PDE inhibitors could serve as a reference to develop new therapeutic strategies.
The genome project of P. falciparum has been completed and can now be used to reveal potential new drug targets. Sequence alignments of the PDEs of P. falciparum revealed 4 orthologues of the human PDE, while sequences in regions important for catalytic activity are highly conserved. PDE5 inhibitors such as sildenafil, vardenafil or tadalafil have been developed for the treatment of erectile dysfunction. These PDE5 inhibitors exhibit different PDE selectivity profiles, in particular in their relative affinities for PDE5, PDE6 (found in photoreceptor cells) and PDE11 (of yet unclear biological function). Tadalafil present a good selectivity profile over PDE6.
The synthesis of tetrahydro-beta-carboline derivatives, as tadalafil, is relatively simple, opening access to a variety of rationally designed structural modifications, permitting what we refer as a “molecular morphing” that would change pharmacological properties through a progressive transition.
The N-methyl group of tadalafil is directed toward a large unoccupied pocket of the active site of the enzyme, permitting the creation of a tadalafil-derived family with enlarged structural diversity through the replacement of the N-methyl group by novel aryl substituants. The effect of these modifications was evaluated in the cell proliferation of asexual blood parasites using an in vitro antimalarial activity assay. Interestingly, treatment with 2 of these analogues resulted in growth inhibition of the parasites with IC50 within the nanomolar range when tested against chloroquino-resistant or -sensitive strains. The most active compounds were tested in vivo, at the dose of 50 mg/kg. Several compounds were found active, and a European patent application was deposited in October 2006.
Using this original “molecular morphing” approach, we have been able to turn PDE5- into P. falciparum PDE -selectivity, and to obtain compounds with in vitro antimalarial activities superior to chloroquine and comparable to artesunate. Our aim is now to produce a pre-clinical drug candidate by optimizing simultaneously multiple properties according to 3 types of requirements:
Our compounds display relatively low water solubility (1-10 μg/mL) and low oral bioavailability in mouse: at a 50 mg/kg dose, plasma concentrations are hardly above IC50. SAR studies show that some positions are available for structural modifications permitting solubilisation without alteration of the in vitro activity. Several point of metabolic fragility have also been identified which will be modified. Stability in the presence of human microsomes will also be addressed.
2 amino acids involved in the interaction between tadalafil and PDE5 are modified in the P. falciparum PDEs. We will exploit these differences to obtain a better selectivity, through modifications of the corresponding area of our molecules. Previously SAR developed at these positions demonstrated their importance to obtain PDE5 inhibition. We also plan to maintain novelty and patentability with reference to previously described tadalafil derivatives.
In collaboration with the group of Jamal Khalife (Inserm U547, Lille – director : Pr Monique Capron), we plan to formally identify the target of our compounds and to test their inhibiting activities by cloning and expressing the 4 P. falciparum PDEs. At the end of this process, we hope to produce compounds active within the nanomolar range with good pharmacokinetic, metabolic and toxicity profiles. In this aspect, we believe that their mechanism of action, related to the one of a marketed drug, will represent a competitive advantage over other strategies base on completely novel mechanisms. We also plan to test our P. falciparum PDE inhibitors with other parasite organisms, such as Trypanosoma, potentially opening new strategies for anti-protozoal chemotherapy.
The work is supported by INSERM, Institut Pasteur de Lille, CNRS, la Région Nord – Pas de Calais, the Ministère de la Recherche, and by the ANR-émergence for the period 2007-2008.